Being able to decide not only whether a micron-scale particle twists but also how much could open new avenues for machine vision and more
Micron-sized “bow ties,” self-assembled from nanoparticles, form a variety of different curling shapes that can be precisely controlled, a research team led by the University of Michigan has shown.
The development opens the way for easily producing materials that interact with twisted light, providing new tools for machine vision and producing medicines.
While biology is full of twisted structures like DNA, known as chiral structures, the degree of twist is locked in—trying to change it breaks the structure. Now, researchers can engineer the degree of twist.
Such materials could enable robots to accurately navigate complex human environments. Twisted structures would encode information in the shapes of the light waves that reflect from the surface, rather than in the 2D arrangement of symbols that comprise most human-read signs. This would take advantage of an aspect of light that humans can barely sense, known as polarization. The twisted nanostructures preferentially reflect certain kinds of circularly polarized light, a shape that twists as it moves through space.
“It is basically like polarization vision in crustaceans,” said Nicholas Kotov, the Irving Langmuir Distinguished University Professor of Chemical Sciences and Engineering, who led the study. “They pick up a lot of information in spite of murky environments.”
Robots could read signs that look like white dots to human eyes; the information would be encoded in the combination of frequencies reflected, the tightness of the twist and whether the twist was left- or right-handed.
By avoiding the use of natural and ambient light, relying instead on circularly polarized light generated by the robot, robots are less likely to miss or misinterpret a cue, whether in bright or dark environments. Materials that can selectively reflect twisted light, known as chiral metamaterials, are usually hard to make—but the bow ties aren’t.
“Previously, chiral metasurfaces have been made with great difficulty using multimillion dollar equipment. Now, these complex surfaces with multiple attractive uses can be printed like a photograph,” Kotov said.
Twisted nanostructures may also help create the right conditions to produce chiral medicines, which are challenging to manufacture with the correct molecular twist.
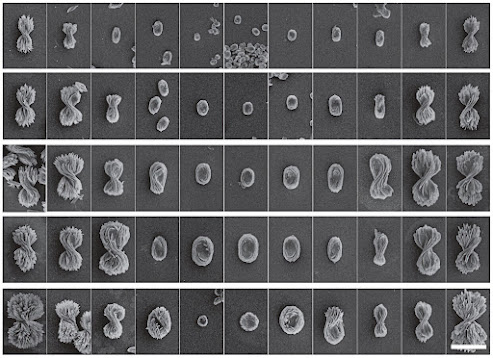
“What hasn’t been seen in any chiral systems before is that we can control the twist from a fully twisted left-handed structure to a flat pancake to a fully twisted right-handed structure. We call this a chirality continuum,” said Prashant Kumar, a U-M postdoctoral research fellow in chemical engineering and first author of the study in Nature.
Kumar tested the bow ties as a sort of paint, mixing them with polyacrylic acid and dabbing them onto glass, fabric, plastic and other materials. Experiments with lasers showed that this paint reflected twisted light only when the twist in the light matched the twist in the bow tie shape.
The bow ties are made by mixing cadmium metal and cystine, a protein fragment that comes in left- and right-handed versions, in water spiked with lye. If the cystine was all left-handed, left-handed bow ties formed, and right-handed cystine yielded right-handed bow ties—each with a candy-wrapper twist.
But with different ratios of left-and right-handed cystine, the team made intermediate twists, including the flat pancake at a 50-50 ratio. The pitch of the tightest bow ties, basically the length of a 360-degree turn, is about 4 microns long—within infrared light’s range of wavelengths.
“Not only do we know the progression from the atomic scale all the way up to the micron-scale of the bow ties, we also have theory and experiments that show us the guiding forces. With that fundamental understanding, you can design a bunch of other particles,” said Thi Vo, a former U-M postdoctoral researcher in chemical engineering.
He worked with Sharon Glotzer, co-corresponding author of the study and the Anthony C. Lembke Department Chair of Chemical Engineering at U-M.
In contrast with other chiral nanostructures, which can take days to self-assemble, the bow ties formed in just 90 seconds. The team produced 5,000 different shapes within the bow tie spectrum. They studied the shapes in atomic detail using X-rays at Argonne National Laboratory ahead of the simulation analysis.
Additional material analysis and contributions to theory were provided by collaborators at U-M, the University of Pennsylvania, the University of Palermo in Italy and Pro Vitam Ltd, Romania. The study was supported by the Office of Naval Research, National Science Foundation and Army Research Office.
Kotov is also the Joseph B. and Florence V. Cejka Professor of Engineering and a professor of chemical engineering and macromolecular science and engineering. Vo is now a professor of chemical and biomolecular engineering at Johns Hopkins University. Glotzer is also the John Werner Cahn Distinguished University Professor of Engineering, the Stuart W. Churchill Collegiate Professor of Chemical Engineering, and a professor of materials science and engineering, macromolecular science and engineering, and physics.
Published in journal: Nature
Source/Credit: University of Michigan
Reference Number: nt031523_01