.gif) |
| A time-elapse video showing how color develops in areas of a specialized polymer that have been placed under strain. Video Credit: Peter Holderness/Caltech |
Stress isn't just the psychological pressure you feel in response to a looming deadline at work. It is also a description of the physical forces pushing, pulling, or twisting an object, structure, or material. Examples of stress include gravity dragging downward on a bridge, wind blowing against the side of a building, or even a waistband drawn taut by a big meal.
With stress affecting literally everything made and used by people, often in damaging ways, it is important to identify when and where it is happening and the extent to which it is occurring. This is not always easy, though, because many materials show no obvious signs of being under stress.
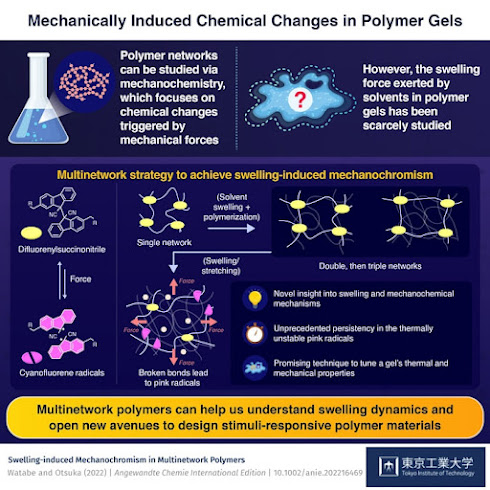
Caltech's Maxwell Robb, an assistant professor of chemistry, has been working to make stress easier to identify through the creation of polymers that change color when a force is applied to them. Now, in a paper published in Nature Chemistry, Robb shows how his team created a new type of these polymers that can be made to change to almost any colors the user wants. This is in contrast to the polymers he had previously developed, which could only change to a single, predetermined color.







.jpg)




.jpg)
.jpg)
.jpg)








.jpg)
