 |
| Artistic depiction of a giant rotor molecule rotating in the solid state. Illustration Credit: Rempei Ando, et al. Angewandte Chemie International Edition. |
Concave, umbrella-like metal complexes provide space to enable the largest molecular rotor operational in the solid-state.
Solid materials are generally known to be rigid and unmoving, but scientists are turning this idea on its head by exploring ways to incorporate moving parts into solids. This can enable the development of exotic new materials such as amphidynamic crystals—crystals which contain both rigid and mobile components—whose properties can be altered by controlling molecular rotation within the material.
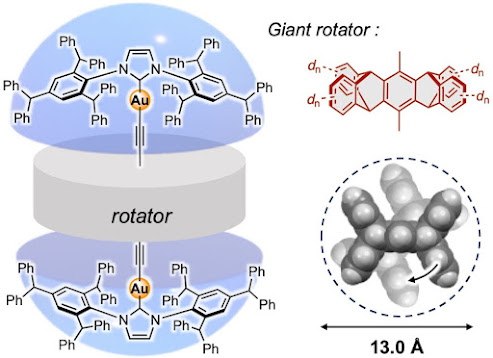
A major challenge to achieving motion in crystals—and in solids in general—is the tightly packed nature of their structure. This restricts dynamic motion to molecules of a limited size. However, a team led by Associate Professor Mingoo Jin from the Institute for Chemical Reaction Design and Discovery (WPI-ICReDD), Hokkaido University has set a size record for such dynamic motion, demonstrating the largest molecular rotor shown to be operational in the solid-state.
A molecular rotor consists of a central rotating molecule that is connected by axis molecules to stationary stator molecules, similar to the way that a wheel and axle are connected to a car frame. Such systems have been previously reported, but the crystalline material in this study features an operational rotor consisting of the molecule pentiptycene, which is nearly 40% larger in diameter than previous rotors in the solid-state, marking a significant advancement.
To enable rotation of such a large molecule, it was necessary to create enough free space within the solid. The team synthesized concave, umbrella-like metal complexes that could shield the rotor molecule from unwanted interactions with other molecules in the crystal. They were able to create sufficient space to accommodate the giant rotor by attaching an especially large, bulky molecule to the metal atom of the stator.
“I got the idea from an egg, which makes a large space and protects its inside with a circular hardcover,” said Jin. “To bring this feature to a molecule, I envisioned encapsulating the rotator space by using bulky concave shaped stators.”
A comparison of experimental and simulated nuclear magnetic resonance spectra of the crystal suggested that the giant molecular rotor rotates in 90-degree intervals at a frequency in the range of 100–400 kHz.
This work expands what is possible for molecular motion in the solid-state. It provides a blueprint for exploring new avenues in the development of amphidynamic crystals, and could lead to the development of new functional materials with unique properties.
“The pentiptycene rotators utilized in this work have several pocket sites,” commented Jin. “This structural feature allows the inclusion of many types of guest compounds including luminophores, which could enable development of highly functional, sophisticated optical or luminescent solid-state materials.”
Funding: This work was supported by the Japan Society for the Promotion of Science (JSPS) KAKENHI grants (JP17H06370, JP17H06372, JP21K14637, JP22K18333, JP22H00318); the Japan Science and Technology Agency (JST) CREST (JPMJCR19R1); the JSPS Fellows (JP22J20934, JP22KJ0116) Researchers; and by the Institute for Chemical Reaction Design and Discovery (ICReDD), which was established by the World Premier International Research Initiative (WPI) of the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Published in journal: Angewandte Chemie International Edition
Source/Credit: Hokkaido University
Reference Number: chm092923_01







.jpg)