The international team from the Peter Doherty Institute for Infection and Immunity (Doherty Institute – a joint venture between the University of Melbourne and the Royal Melbourne Hospital), the University of Queensland, Griffith University, the University of Adelaide, and St Jude Children’s Research Hospital (USA), found how to repurpose a molecule called PBT2 - originally developed as a potential treatment for disorders such as Alzheimer's, Parkinson’s and Huntington’s diseases – to break bacterial resistance to commonly used frontline antibiotics.
Led by University of Melbourne Professor Christopher McDevitt, a laboratory head at the Doherty Institute, this discovery may see the comeback of readily available and cheap antibiotics, such as penicillin and ampicillin, as effective weapons in the fight against the rapidly rising threat of antibiotic resistance.
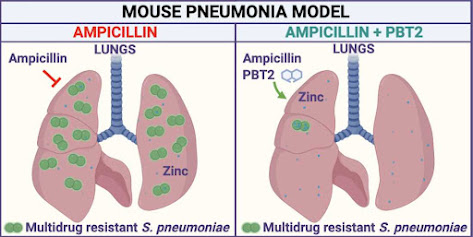
In a paper published today in Cell Reports, Professor McDevitt and his collaborators described how they discovered a way to break bacterial drug resistance and then developed a therapeutic approach to rescue the use of the antibiotic ampicillin to treat drug-resistant bacterial pneumonia caused by Streptococcus pneumoniae in a mouse model of infection.
Last year the World Health Organization (WHO) described antibiotic resistance as one of the greatest threats to global health, food security, and development. Rising numbers of bacterial infections – such as pneumonia, tuberculosis, gonorrhoea, and salmonellosis – are becoming harder to treat as the antibiotics used against them are becoming less effective.
Professor McDevitt’s previous research into bacterial antibiotic resistance using zinc ionophores led to collaborations with University of Queensland’s Professor Mark Walker and Griffith University's Professor Mark von Itzstein from the Institute for Glycomics.
“We knew that some ionophores, such as PBT2, had been through clinical trials and shown to be safe for use in humans,” Professor von Itzstein said.
Professor Walker said: “As a group, we realized that if we could repurpose these safe molecules to break bacterial resistance and restore antibiotic efficacy, this would be a pathway to a therapeutic treatment. What we had to do was show whether PBT2 broke bacterial resistance to antibiotic treatment without leading to even greater drug resistance."
“We focused on bacterial pneumonia and the most commonly used antibiotics. We thought that if we could rescue frontline antibiotics and restore their use for treating common infections, this would solve a global problem,” Professor McDevitt added.
Crucially important was the research from Professor McDevitt’s group that led to knowing how and where treatment could be most effective.
“We knew from earlier research that the immune system uses zinc as an innate antimicrobial to fight off infection. So, we developed our therapeutic approach with PBT2 to use the body’s antimicrobial zinc to break antibiotic resistance in the invading bacteria,” he said.
“This rendered the drug-resistant bacteria susceptible to the antibiotic ampicillin, restoring the effectiveness of the antibiotic treatment in the infected animals.”
Professor von Itzstein says this discovery has the potential to provide a cost-effective and readily available treatment to life-threatening infections such as community-acquired bacterial pneumonia, which poses a serious public health risk.
“In Australia, this is of particular importance for Aboriginal and Torres Strait Islander communities who are four times more susceptible to bacterial pneumonia and 11 times more likely to succumb to infection,” Professor Walker added.
Professor McDevitt said the next steps they were working towards were collecting the data required for a clinical trial of PBT2 in combination with antibiotics.
“We also want to find other antibiotic-PBT2 combinations that have therapeutic potential for treatment of other bacterial infections,” he said.
“Our work shows that this simple combination therapy is safe, but the combinations require testing in clinical trials. What we need now is to move forward with further testing and pharmacology.”
Source/Credit: University of Melbourne
med011122_04







.jpg)