A new way of reprogramming our immune cells to shrink or kill off cancer cells has been shown to work in the otherwise hard to treat and devastating skin cancer, melanoma. The University of Bristol-led discovery, published in Advanced Science today [31 October], demonstrates a new way to clear early stage pre-cancerous and even late-stage tumor cells.
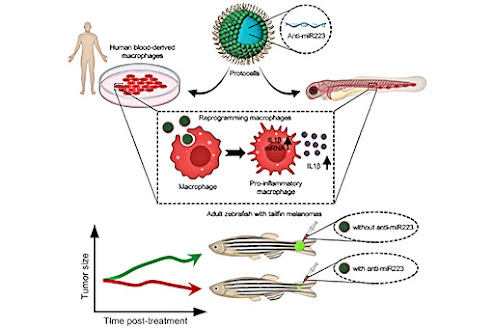
Using miniature artificial capsules called protocells designed to deploy reprogramming cargoes that are taken up by inflammatory cells (white blood cells), the scientists show they were able to transform these cells into a state that makes them more effective at slowing down the growth and killing of melanoma cells. They showed that this was possible for both animal and human immune cells.
The study is the first to test the capacity of a protocell to deliver cargoes for reprogramming immune cells and offers a promising novel target for the development of cancer immunotherapies.
Paul Martin, Professor of Cell Biology in the School of Biochemistry at the University of Bristol and one of the study's lead authors explained what happens when our immune system comes into contact with cancer cells: "Our immune cells have a surveillance capacity which enables them to detect pre-cancerous cells arising at any tissue site in the body. However, when immune cells encounter cancer cells, they are often subverted by the cancer cells and instead tend to nourish them and encourage cancer progression. We wanted to test whether it might be possible to reprogram our immune system to kill these cells rather than nurture them."
First, the team tested the proof of concept in zebrafish larvae which are used due to their translucency, allowing researchers to watch inflammatory immune cells interact with cancer cells in ways not possible in our own tissues.
Protocells loaded with anti-miR223 molecules that bind to and interfere with signaling machinery in the inflammatory immune cells and work by effectively prolonging their pro-inflammatory state, were shown to drive altered immune cell-cancer cell interactions, slowing the growth of cancerous cells and driving increased tumor cell death in the larvae.
To find out whether this approach might be upscaled as a feasible therapeutic strategy for shrinking larger, more established, and growing cancers, the experiment was repeated in adult fish with tailfin melanomas, showing this approach significantly inhibited melanoma cell growth.
To fully investigate the feasibility of using protocells to deliver 'reprogramming' anti-miR223 cargoes in humans, the experiment was conducted again using an in vitro assay with primary human immune cells from the Toye lab, also in Bristol’s School of Biochemistry. Results from this experiment demonstrated that the protocells were able to effectively deliver and reprogram human immune cells toward a more persistent pro-inflammatory and potentially anti-cancer state.
Professor Stephen Mann from Bristol's School of Chemistry and the Max Planck Bristol Centre for Minimal Biology added: "Our results highlight the therapeutic benefits of harnessing host immunity to eradicate cancers and demonstrate the feasibility of using protocells to deliver cargoes for reprogramming innate immune cells. While our experiments in zebrafish are early pre-clinical studies, our results indicate that the same is possible for human immune cells, at least in vitro, and can be similarly reprogrammed to suppress cancer growth."
The study was supported by grants from the Spanish Rafael del Pino Foundation, a Bristol Cancer Bequest, EU Marie Curie fellowship funded through HORIZON 2020, BBSRC (BrisEngBio), Wellcome, Elizabeth Blackwell Institute, the European Research Council (ERC) and Cancer Research UK (CRUK).
Source/Credit: University of Bristol
bio103122_02

.jpg)





.jpg)